Why RWE Data Curation Needs a Domain-Led Approach
Posted on: October 13th 2025
Real-world evidence (RWE) has become a critical tool for transformation in pharmaceutical and life sciences. By drawing on diverse life sciences data sources such as electronic health records (EHRs), patient registries, wearable devices, and patient-reported outcomes, RWE delivers insights far beyond what traditional clinical trials offer. It enables companies to understand how therapies perform in everyday practice and provides regulators, payers, and clinicians with evidence that reflects real-world patient experiences.
This opportunity, however, comes with a significant challenge. The deluge of structured and unstructured healthcare data has created a complex environment where information is fragmented, inconsistent, and often unreliable. Traditional clinical data management systems or generic AI-driven pipelines work well for scale but usually fail to capture therapeutic nuance or regulatory requirements. As a result, RWE risks being incomplete, biased, or untrustworthy unless guided by domain-led intelligence.
To unlock its full potential, pharma and healthcare organizations must adopt curation strategies integrating automation with clinical, regulatory, and therapeutic expertise. This is
where Straive’s healthcare data strategy and domain-led curation advantage make a measurable difference.
Why RWE Data Matters
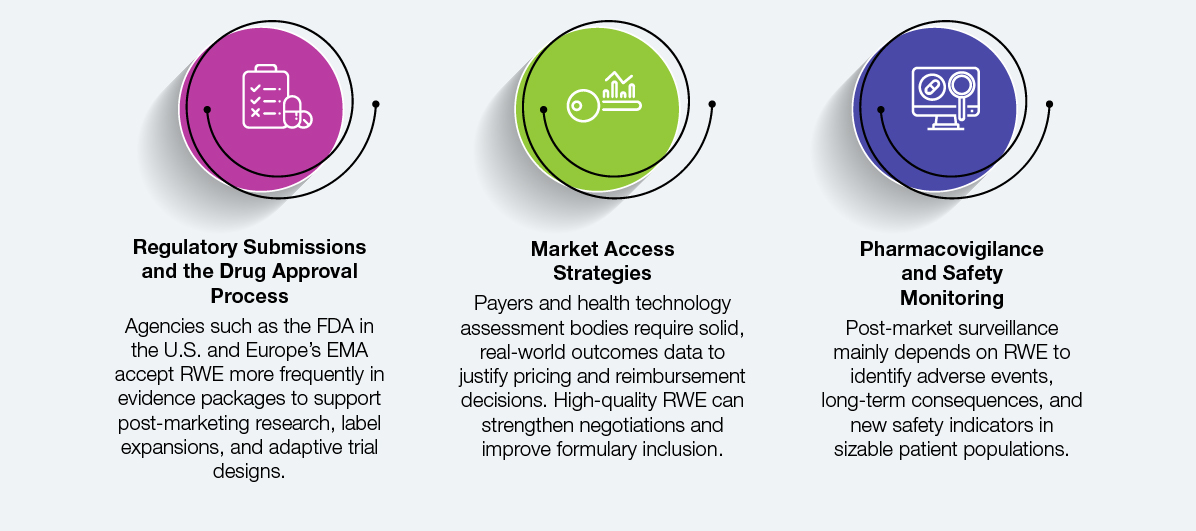
RWE’s influence is growing across the entire drug development and commercialization lifecycle. Its applications are now central to several mission-critical functions, such as:
In each case, the quality and reliability of curated evidence directly determine the outcome. If EHR data analysis or patient registries are riddled with inconsistencies, approval timelines may be delayed, payer confidence may be undermined, or critical safety signals may be overlooked. On the other hand, carefully chosen, domain-led RWE promotes improved business and patient outcomes, speeds up reviews, and increases trust.
The Complexity of RWE Data
While the diversity of RWE makes it rich, that same diversity creates significant complexity. Structured datasets and unstructured healthcare data from various ecosystems are among the sources:
- EHRs and claims data often vary by coding standards (ICD, SNOMED, MedDRA) and may contain incomplete or duplicate records.
- Patient registries differ in data governance models and completeness, sometimes lacking detailed clinical descriptors.
- Digital health devices and apps generate vast amounts of continuous data, but much of it lacks the data standardization required for regulatory use.
- Global datasets introduce multilingual challenges and local variations in practice, complicating data interoperability.
Stringent legal requirements regarding data governance, consent, and privacy worsen these problems. Curation procedures must be hardwired to comply with HIPAA, GDPR, and other frameworks. In the absence of robust data interoperability and data standardization, organizations risk developing separate silos rather than cohesive, decision-useful datasets.
The Limitations of a Generic Data Approach
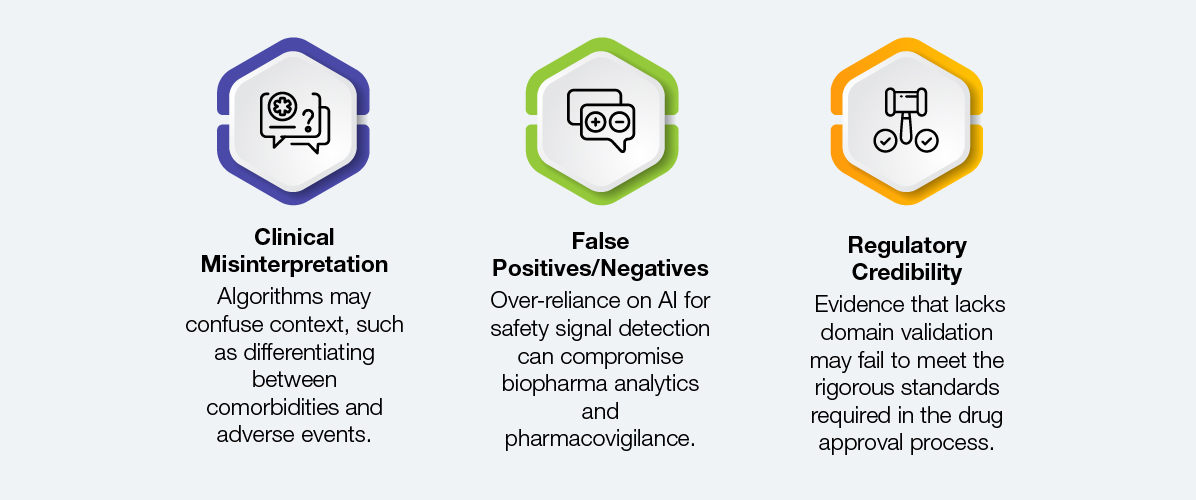
While many pharmaceutical and life sciences companies use AI-only pipelines or generic data cleaning to address RWE complexity, these methods are ineffective in high-stakes decision-making.
Although generic health informatics platforms maximize productivity, they rarely meet the accuracy and compliance requirements for regulatory-grade RWE. At first glance, insights might appear comprehensive, but upon closer examination, they may fall apart, wasting time and money.
Why a Domain-Led Approach is Essential
A domain-led approach addresses these limitations by embedding contextual expertise at every step of RWE data curation. It ensures that data is precise, clear, clinically significant, ready for regulation, and aligned with actual medical practice.
Key advantages include:
- Accurate data standardization and interoperability, harmonizing fragmented datasets without losing therapeutic nuance.
- Improved safety and efficacy insights, as experts validate which clinical signals are significant.
- Compliance alignment, embedding data governance frameworks, and regulatory expectations into workflows.
- Bias reduction, ensuring RWE reflects diverse populations and avoids distorted conclusions.
Thanks to this combination of technical and clinical oversight, RWE’s value is unlocked across research, regulation, and care delivery. This oversight ensures that RWE is both practically useful and scientifically credible.
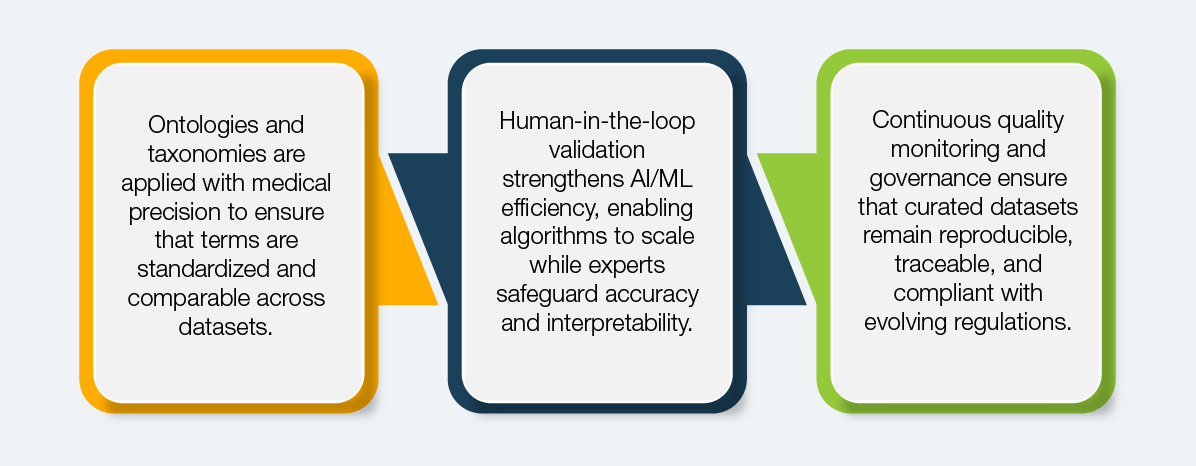
How Domain-Led Curation Works
Domain-led curation is built on technology, expertise, and process synergy.
This method speeds up the curation process and generates outputs that pass scrutiny during payer assessments and regulatory reviews. For biopharma analytics, it ensures that insights derived from RWE are actionable, trustworthy, and directly supportive of innovation and patient care.
Straive’s Domain-Led RWE Curation Advantage
Straive applies its expertise in clinical data management, health informatics, and healthcare data strategy to help life sciences companies transform fragmented Real World Data into high-quality, regulator-ready Real World Evidence.
Straive’s differentiators include:
- Experienced curators with backgrounds in pharma, clinical research, and regulatory affairs who understand therapeutic nuances.
- AI + human expertise integration, combining scale with contextual precision.
- Demonstrated ability to achieve data interoperability and data standardization across highly fragmented sources.
- Strong focus on compliance and data governance, ensuring privacy, traceability, and publishing integrity.
Straive provides RWE solutions through its domain-led model that help organizations strengthen their market access strategies, accelerate the drug approval process, and build scientific credibility. By embedding expertise into every curation stage, Straive enables pharma companies to unlock the value of life sciences data for regulators, payers, and clinicians.
Conclusion: Unlocking the True Value of RWE
The actual value of RWE lies not just in the breadth of real-world data but also in its ability to curate it with precision, compliance, and contextual understanding. Although generic approaches might be faster, they risk producing biased, incomplete, or unsuitable outputs for regulatory use without domain expertise.
A domain-led approach ensures that RWE is reliable, compliant, and clinically relevant. This approach is no longer optional; it is now necessary for organizations managing intricate safety monitoring, market access strategies, and the drug approval process.
Straive combines domain expertise, data governance, and advanced biopharma analytics to transform fragmented unstructured healthcare data into decision-ready insights. Straive’s proven AI plus human intelligence model enables pharmaceutical companies to confidently use RWE, generating evidence that payers value, regulators trust, and clinicians use to enhance patient care.
Explore Straive’s life sciences solutions to see how domain-led curation can unlock the full potential of your real-world evidence.
About the Author

Santosh Shevade is a Principal Data Consultant at Gramener – A Straive Company. With deep expertise in healthcare strategy, digital health, and clinical development and operations, he has supported nearly 50 clinical development programs across all clinical phases. His experience spans advanced analytics solution design for pharmaceutical companies, mHealth implementation, and AI applications in healthcare. Previously at Novartis and Johnson & Johnson, Santosh led global clinical development teams and streamlined data review processes for major regulatory submissions. A certified MBTI trainer and leadership coach, he serves as visiting faculty at ISB Hyderabad and Welingkar Institute, focusing on healthcare technology innovation and biopharma strategy.
Share with Friends:
We want to hear from you
Leave a Message
Our solutioning team is eager to know about your
challenge and how we can help.