Part 3: How to Automate Drug Development with an AI-Native Approach?
Posted on: May 5th 2025
The Most Pressured Stages in Drug Development
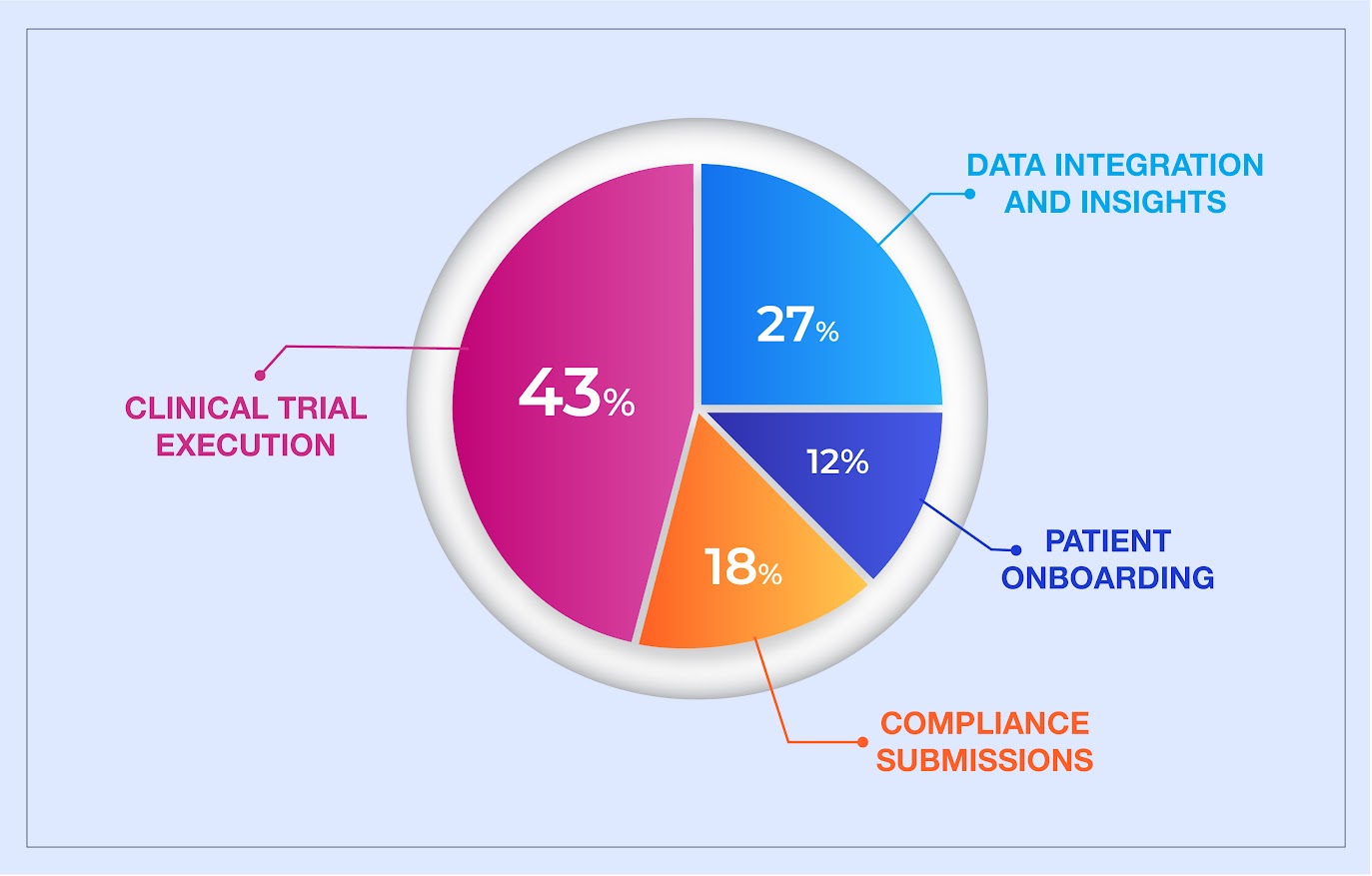
Every stage of clinical development brings its own challenges, but some pinch points are felt more sharply than others. To understand where the urgency is highest, we asked our network of industry professionals through a LinkedIn poll.
Check out their responses in the following image:
These responses echo what we often hear from pharma teams in the field: the real pressure does not just show up in one place. It builds across trials, systems, teams, and timelines. Whether it is delayed site reporting, siloed data, or regulatory backlogs, the need for speed is constant. With AI-native frameworks, that pressure becomes a solvable problem, not just a persistent pain point.
Proven Outcomes and Why Straive Leads the Way
AI-native drug development is more than just a catchphrase these days. It is real, here to stay, and already giving forward-looking pharmaceutical companies quantifiable outcomes. While in Part 1 of this blog series, we covered the why—and in Part 2, we unpacked the how—this penultimate part showcases the what: the actual business and scientific value unlocked by AI-native frameworks when they are operationalized at scale.
At Straive, we have guided global pharma clients beyond pilot projects and digital token efforts to fully integrate AI-powered ecosystems, such as cutting costs, timelines, and manual friction across the clinical value chain. This is not about automating a few processes; it’s about enabling drug development from protocol design to regulatory submission by facilitating a full-stack transformation.
Here’s an insider look at how this plays out in practice.
Transforming Clinical Trial Oversight with AI-Powered Insights
One major pharmaceutical company faced delays and inefficiencies in managing clinical trials across multiple sites. Straive deployed an AI-powered Trial Operations Insights platform, enabling unified reporting, predictive risk analytics, and a centralized operations cockpit. The result? Over $2.4 million in savings, a 75% reduction in open issues, and significantly improved trial visibility within just six months.
Accelerating Regulatory Responses with GenAI-Powered HAQ Automation
Similarly, a European pharma leader struggling with the rising volume of Health Authority Queries (HAQs) turned to Straive. By implementing a GenAI-powered HAQ response assistant, we helped cut HAQ turnaround time by more than 50%, improved response consistency, and eased the regulatory workload for teams, enabling faster, smoother submissions.
Scaling Biomedical Annotation for Faster, Smarter Drug Discovery
In the race for AI-driven drug discovery, data quality is everything. A global life sciences company partnered with Straive to scale biomedical annotation using our hybrid AI-automation model. Achieving over 80% automation and 90% accuracy, the solution accelerated R&D initiatives, enabling faster training of high-quality AI models across multiple therapeutic areas.
Each of these success stories underscores the transformative power of AI when combined with expert-driven workflows. At Straive, we’re committed to helping life sciences organizations unlock new levels of operational efficiency, data quality, and innovation.
Sraive is your Partner built for Pharma Transformation
The aforementioned case studies are all excellent illustrations of what can be achieved when artificial intelligence is integrated into drug development rather than merely tacked on as a post-it note. However, these outcomes also point to something crucial: how Straive delivers transformation differently.
Straive is designed for end-to-end operationalization, whereas many providers only work on model development or proof-of-concept. Why our clients trust us:
Expertise That Speaks
With 600+ life sciences professionals and 38+ PhDs, our teams know the science, compliance, and subtleties of the pharmaceutical industry and AI.
Domain-Trained AI Stack
Our LLM Foundry, biomedical data pipelines, and domain-tuned copilots ensure that every insight is contextually accurate, auditable, and scalable.
Expert-in-Loop Frameworks
We design every solution with human oversight baked in. This ensures compliance, fosters trust and builds confidence in AI adoption—especially in sensitive, regulated settings.
Outcome-First Mindset
Whether it’s reducing trial costs, cutting submission timelines, or scaling data annotation, we focus on impact—not just innovation.
The Bottom Line: AI-Native Is No Longer Optional
As competitors race ahead with intelligent trial design, adaptive protocols, and more innovative regulatory processes, pharma companies can no longer afford to stay stuck in outdated workflows or experiment with siloed AI pilots. The future belongs to those who can make AI-native development a repeatable, scalable reality.
Straive is that partner
With our AI-native approach, global life sciences organizations are building faster, smarter, and more efficient drug development pipelines, improving time to market, reducing costs, and better-serving patients.
Get in Touch With Us Today
Whether you are ready to scale your AI journey or exploring where to begin, Straive can help you operationalize AI where it matters most—in real-world pharma environments.
Contact us today to see how we can help your teams move from automation to transformation.
About the Author

Santosh Shevade is a Principal Data Consultant at Gramener – A Straive Company. With deep expertise in healthcare strategy, digital health, and clinical development and operations, he has supported nearly 50 clinical development programs across all clinical phases. His experience spans advanced analytics solution design for pharmaceutical companies, mHealth implementation, and AI applications in healthcare. Previously at Novartis and Johnson & Johnson, Santosh led global clinical development teams and streamlined data review processes for major regulatory submissions. A certified MBTI trainer and leadership coach, he serves as visiting faculty at ISB Hyderabad and Welingkar Institute, focusing on healthcare technology innovation and biopharma strategy.
Share with Friends:
We want to hear from you
Leave a Message
Our solutioning team is eager to know about your
challenge and how we can help.