Optimizing Modular Content
Creation for Pharma Teams
Posted on: December 26th 2025
Pharmaceutical organizations manage an enormous volume of scientific, regulatory, and medical information, content that must remain consistent, compliant, and globally aligned. Yet many teams still work inside traditional document-heavy workflows marked by manual drafting, email-led reviews, and disconnected repositories. These processes create friction at every stage. One change in safety language may trigger revisions across dozens of assets; a simple terminology update can send teams hunting through old folders, trying to determine which version is truly the latest.
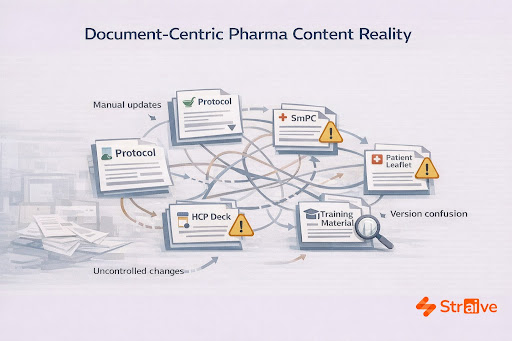
This is the reality many organizations face: not a lack of expertise, but a system of content operations that cannot keep up with the pace and precision required today. As global portfolios expand and regulatory expectations evolve, teams need a scalable, structured, and reliable method for managing scientific information. That shift is what drives the growing adoption of modular content models, and it is where Straive’s capabilities make a measurable difference.
From the outset, Straive works with life sciences organizations to move beyond document-centric creation toward structured, component-based ecosystems supported by metadata, governance, and intelligent workflow design. Modular content becomes more than a technical model; it becomes an operational foundation for compliance, consistency, and scalability.
The Persistent Pharma Content Bottleneck
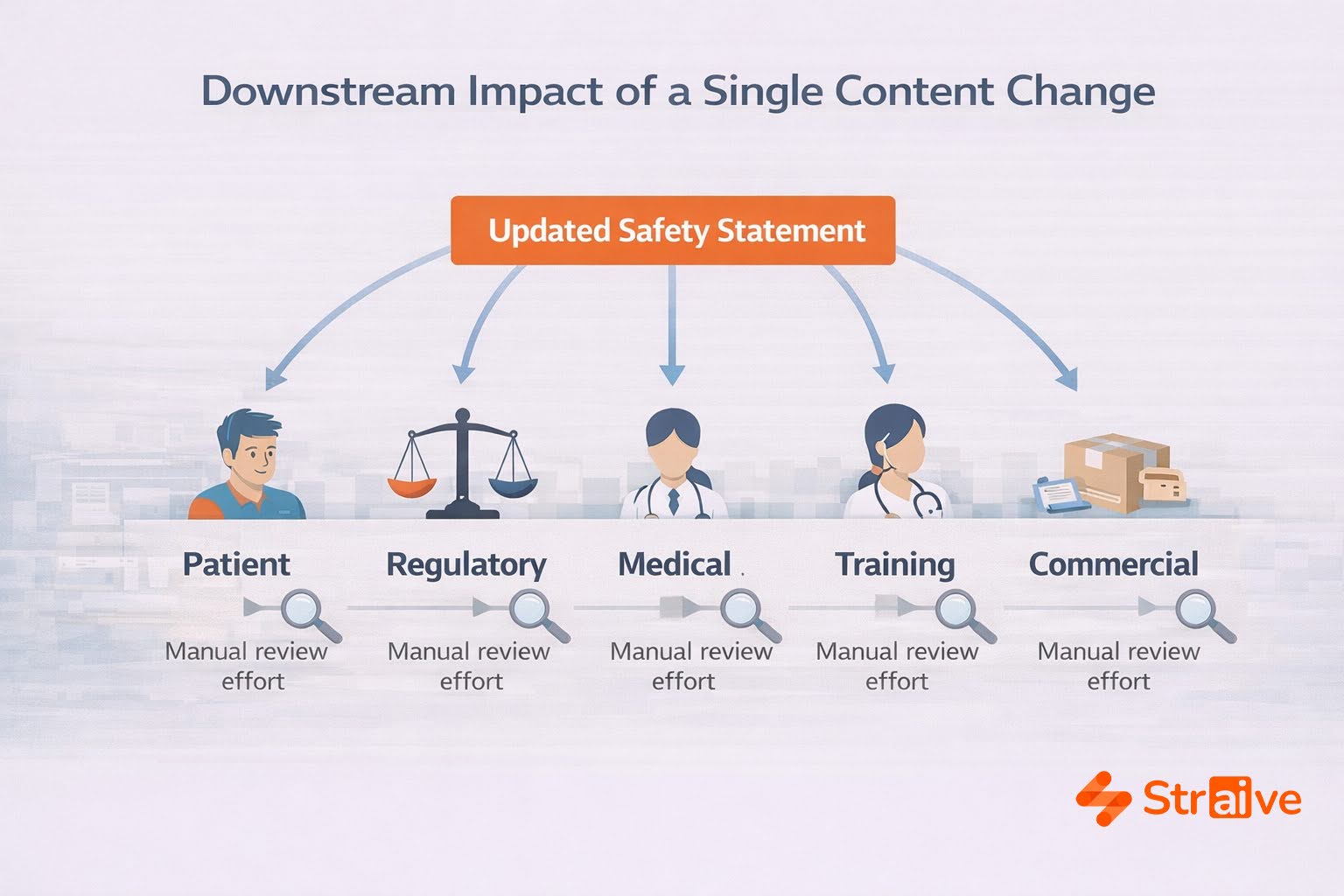
The most significant challenge in pharma content workflows is not producing the first draft. It is maintaining alignment across countless downstream documents. A single update to an adverse-event description or dosing instruction can affect:
- patient materials
- labeling submissions
- HCP-facing content
- internal references
- training materials
- commercial assets
Under traditional workflows, each document must be opened, edited, reviewed, and reconciled manually. This increases cycle time and introduces variation, in terminology, phrasing, and interpretation, that regulatory teams must then correct.
Compounding this issue, content often lives in isolated systems. Regulatory teams maintain one repository, medical affairs another, regional teams a third. Without a unified taxonomy or governance model, version drift becomes inevitable.
Straive frequently encounters organizations where five versions of the same safety statement appear across different departments. Nothing is technically “wrong,” yet nothing is fully aligned. These micro-variations become major risks when they appear in submissions or patient-facing materials.
Modular content eliminates this fragmentation by shifting the unit of management from documents to components. Each approved statement exists once—validated, version-controlled, and published from a governed source.
Understanding Modular Content in Pharma
Modular content breaks information into discrete, reusable components, each representing a single scientific or regulatory idea. These modules often include:
- mechanism-of-action summaries
- standardized safety language
- dosage and administration statements
- medical claims and efficacy descriptions
- patient guidance segments
Each module is enriched with metadata that describes:
- approval status
- therapeutic context
- usage constraints
- relationships to other modules
- global vs. regional applicability
Metadata becomes the backbone of modular systems. It dictates how content should be assembled, where it can appear, and which rules govern its use.
Writers no longer build documents from scratch; they assemble them from validated components. When safety language changes, updating one module updates every dependent asset automatically. This eliminates redundancy, accelerates reviews, and ensures scientific and regulatory consistency across functions and markets.
Straive supports clients in designing these components and the metadata structures that control how they flow across the organization.
Key Benefits of Modular and Reusable Content Models
1. Faster, More Predictable Reviews
Because modules are pre-approved, reviewers focus on verifying context—not rewriting every line. Regulatory, medical, and legal teams consistently report faster cycles and fewer escalations once modular content models are adopted.
2. Stronger Scientific and Regulatory Consistency
Validated, centrally controlled modules ensure that terminology, claims, safety statements, and dosing instructions remain harmonized across communication channels.
3. Clearer Compliance and Auditability
Modular systems capture full lineage:
Who created the module, how it has evolved, and which assets rely on it.
This traceability strengthens readiness for inspections and internal audits.
4. Higher Operational Efficiency
Teams spend less time re-drafting and reconciling documents and more time on scientific interpretation, content strategy, and medical accuracy.
5. Better Global Scalability
Localization becomes easier and more reliable.
When global teams update a module, regional markets inherit the change automatically, reducing translation drift and ensuring cross-market alignment.
These benefits are not theoretical; they are the operational outcomes Straive sees repeatedly in modular content implementations.
The Technology Foundation Behind Modular Content
A modular ecosystem succeeds only when supported by a strong technological base. Straive helps organizations build this foundation through:
Structured Authoring Environments
Templates guide writers to select approved components instead of drafting free text.
XML-Driven, Component-Based Architectures
These ensure tagging, assembly, governance, and version control are consistent and machine-readable.
Metadata Frameworks and Controlled Vocabularies
Metadata functions as the operational logic of the ecosystem—governing usage rules, classification, relationships, and lifecycle management.
AI-Enabled Content Validation
Straive’s domain-trained AI modules detect duplication, enforce terminology consistency, and flag deviations from approved safety or efficacy language.
Enterprise Integrations
Clients use Veeva Vault, regulatory information management (RIM) systems, structured authoring tools, and global content repositories.
Straive integrates modular content workflows into these platforms so content remains consistent wherever it is created or consumed.
Technology alone is not enough; design and governance determine success. Straive brings all three together.
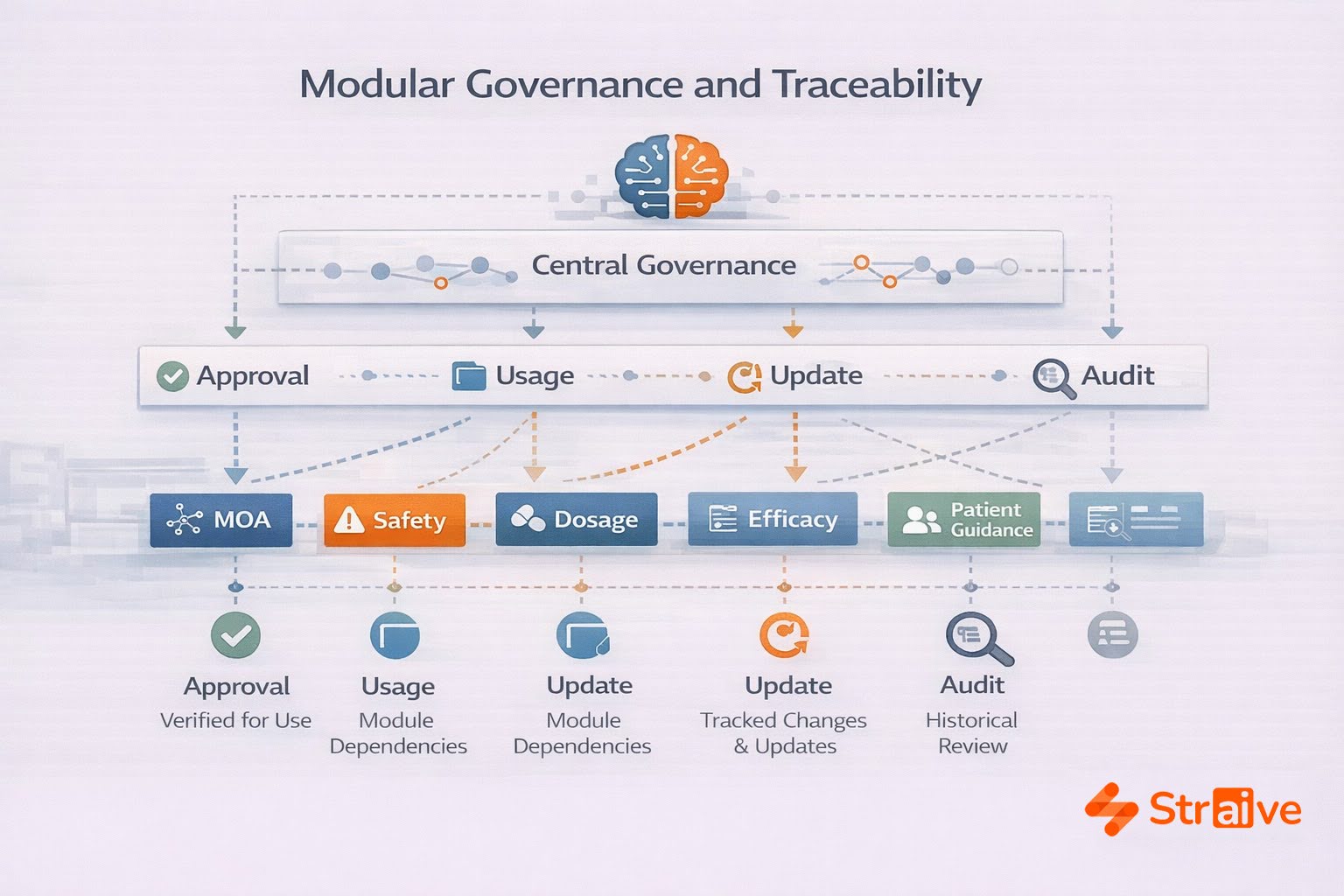
Governance, Traceability, and Quality Control
Modular content requires stronger governance—not less.
Straive works with organizations to implement:
- metadata taxonomies
- module-level approval workflows
- lineage and dependency tracking
- controlled vocabularies and allowed terms
- multilingual governance frameworks
Traceability ensures that when a module changes, downstream implications are understood immediately. This visibility is critical for maintaining regulatory confidence, especially during labeling updates, clinical communication changes, or submissions involving multiple regions.
Straive also supports multilingual modular ecosystems, enabling teams to maintain global consistency while adapting components for regional cultural or regulatory needs.
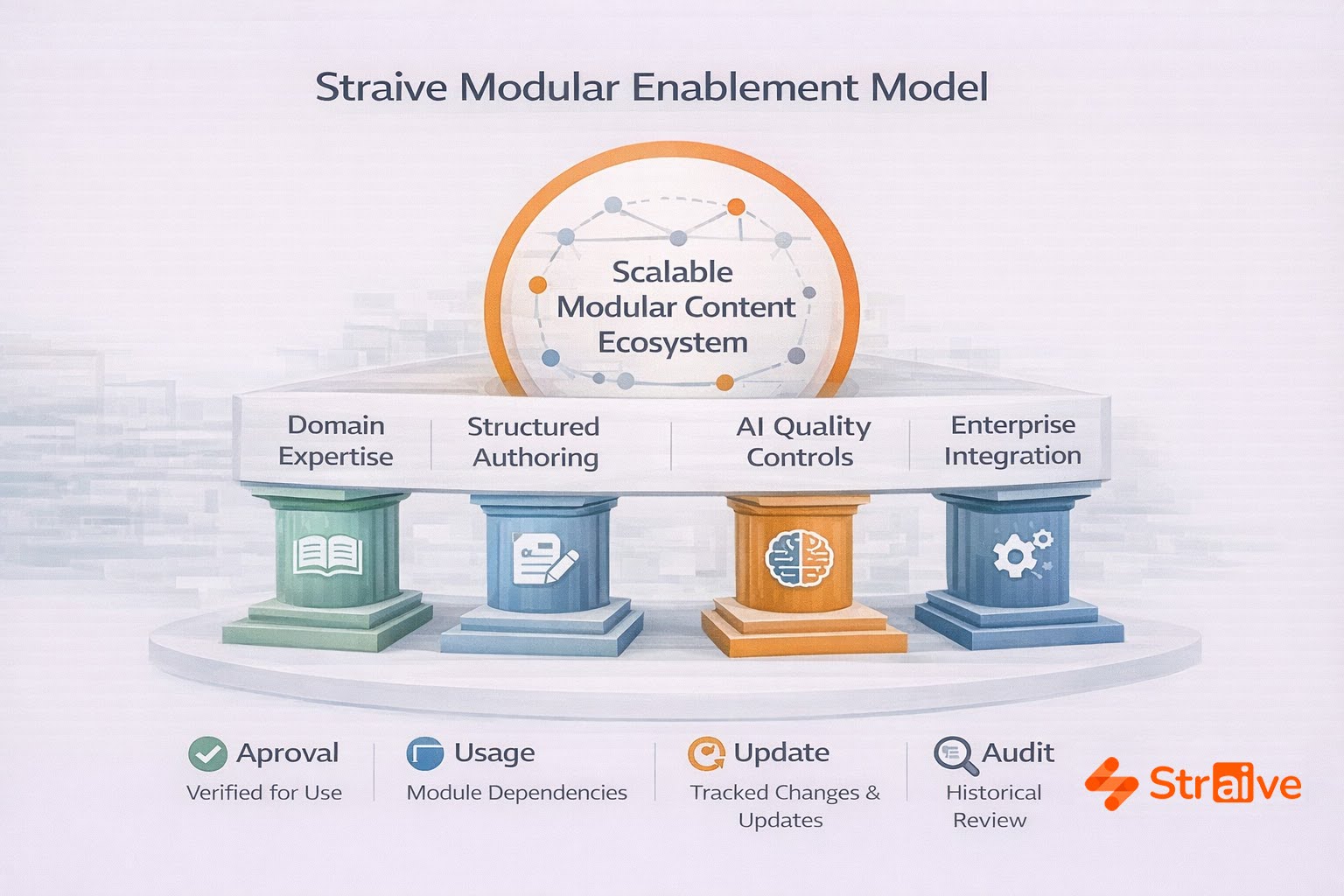
How Straive Enables Modular Content Frameworks
Straive differentiates itself through an integrated approach that combines technology, domain expertise, and operational design.
1. Domain Expertise Across Regulatory, Medical, and Commercial Content
Straive’s teams understand CTDs, CCDS updates, label components, medical narratives, and commercial claims, giving clients confidence that modules reflect regulatory and scientific expectations.
2. Structured Content Authoring Built for Life Sciences
Straive designs component libraries, metadata schemas, and content models that align with therapeutic, regulatory, and global requirements.
3. AI-Enabled Quality and Reuse Detection
Domain-trained AI modules identify duplication, validate terminology, and enforce consistency across versions and markets.
4. Seamless Integration With Enterprise Systems
Straive configures modular workflows across Veeva Vault, RIM tools, structured authoring platforms, and downstream distribution systems.
5. Scalable Content Engineering and Workflow Optimization
Taxonomy development, metadata governance, component mapping, and change-management support ensure organizations can adopt modular content without operational disruption.
This combination of expertise and engineering capability makes Straive a trusted partner in modular transformation.
Conclusion: The Shift Toward Dynamic, Scalable Content Ecosystems
Pharma’s reliance on long-form documents and manual versioning cannot keep pace with global complexity. Modular content offers a scalable path forward: faster reviews, stronger consistency, better governance, and operational efficiency across markets and functions.
The future of pharmaceutical communication will not be document-first but component-first, driven by structured content, metadata, and intelligent workflows.
With deep regulatory expertise, AI-enabled content intelligence, and proven experience in structured authoring and metadata governance, Straive enables organizations to operationalize modular content at scale, turning scientific and regulatory information into an asset that is accurate, reusable, and globally aligned.

Santosh Shevade is a Principal Data Consultant at Gramener – A Straive Company. With deep expertise in healthcare strategy, digital health, and clinical development and operations, he has supported nearly 50 clinical development programs across all clinical phases. His experience spans advanced analytics solution design for pharmaceutical companies, mHealth implementation, and AI applications in healthcare. Previously at Novartis and Johnson & Johnson, Santosh led global clinical development teams and streamlined data review processes for major regulatory submissions. A certified MBTI trainer and leadership coach, he serves as visiting faculty at ISB Hyderabad and Welingkar Institute, focusing on healthcare technology innovation and biopharma strategy.