Breaking Down Data Silos: A Pathway to Enhanced Clinical Insights in Pharma
Posted on: September 18th 2025
Fragmented data silos across R&D, clinical trials, and regulatory departments have emerged as a major obstacle to drug development. Isolated datasets delay cross-functional collaboration, slow drug development timelines, and prevent the extraction of actionable insights from years of valuable research.
These silos lead to repeated experiments, wasted resources, and missed opportunities, increasing operational inefficiency and inflating costs—drug development now averages over $2.2 billion per successful asset. In an industry driven by rapid advancement, unconnected data not only slows progress but also negatively impacts go-to-market and patient outcomes.
Breaking down these data silos is essential for leveraging the full value of clinical data and insights. This blog explores why clinical data silos persist, how to overcome them, and the benefits of a connected, data-driven ecosystem.
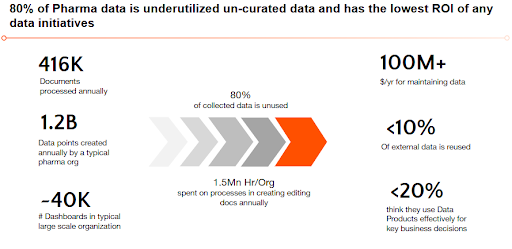
Why Data Silos Persist in Pharma?
Despite the industry’s push toward digital transformation, several deep-rooted structural and operational factors continue to reinforce data silos in pharma. These include:
- Technical Integration Challenges: Fragmented data sources, proprietary extraction tools, and complex modeling requirements make integrating new data or modifying existing systems costly and time-consuming.
- Complex, Global Value Chain: The pharmaceutical value chain spans discovery, manufacturing, distribution, and pricing, often across multiple regions. Poor historical communication between units reinforces functional and geographic silos.
- Prolonged Development Cycles: With drug development spanning 7–9 years, staff turnover, shifting priorities, and changing project teams disrupt continuity and hinder consistent data sharing.
- Resource and Budget Limitations: Budget constraints and competing priorities delay investments in advanced integration platforms or modernized data infrastructure, allowing silos to remain entrenched.
The Strategic Need for Pharma Data Integration
Connecting data across all functions enables end-to-end visibility, empowering evidence-based decision-making, faster innovation cycles, and proactive risk management by bridging the unseen gap between data and insights.
Deloitte’s 2025 insights highlight that AI investments across the pharma value chain could boost revenue by up to 11% and yield up to 12% in cost savings for pharmaceutical and life sciences organizations—but only if supported by transformative, enterprise-wide digital integration rather than isolated, piecemeal adoption.
For more on how AI-powered automation accelerates drug development, see Straive’s AI-Native Drug Development blog
By breaking down these silos, pharma companies can transform raw information into actionable intelligence, enhancing efficiency, ensuring compliance, and accelerating the delivery of life-changing treatments.
Breaking Down Pharma Data Silos: Key Solutions
- Clinical Data Asset Curation and CTMS Optimization: Implementing data integration frameworks anchored in pharma-specific standards such as Clinical Data Interchange Standards Consortium (CDISC), Study Data Tabulation Model (SDTM), Analysis Data Model (ADaM), and Operational Data Model (ODM) ensures consistent structuring of clinical trial datasets. Straive leverages these capabilities to bridge functional and regional gaps—enabling seamless cross-platform exchange, integration monitoring, and compliance-ready data pipelines.
- Scalable Cloud & Unified Data Repositories: Adopting cloud-native platforms and enterprise data lakes allows organizations to integrate legacy and real-time datasets into a single, secure environment. Straive’s cloud engineering and centralized data lake solutions provide role-based access for stakeholders, enhancing operational agility, accelerating decision-making, and enabling seamless collaboration across the pharma value chain.
- AI-Powered Data Harmonization: Leveraging advanced AI, NLP, and data annotation helps cleanse, standardize, and enrich fragmented datasets—improving accuracy, consistency, and revealing hidden insights. Straive applies these capabilities across R&D, clinical, regulatory, and commercial data streams, enabling organizations to break down pharma data silos and accelerate evidence generation.
- Governance-Driven Metadata Management: Establishing strong governance frameworks is essential to ensure regulatory-grade data integrity, traceability, and security. Through solutions like Regulatory Documentation Creation and Data Privacy, Security & GxP Compliance, Straive helps break down pharma data silos by enabling accurate, audit-ready datasets—facilitating safe, efficient sharing of clinical, regulatory, and clinical data across teams.
From Siloed to Streamlined: Business Benefits of Data Integration
- Faster, Evidence-Based Decisions: Integrating data across R&D, regulatory, and commercial operations accelerates decision-making by delivering real-time, unified insights. Modern data platform, providing seamless access to clinical, regulatory, and real-world intelligence, helps teams act faster and more confidently.
- Improved clinical trial efficiency: Clinical trial data integration harmonizes clinical trial management systems (CTMS), electronic data capture (EDC), and real-world data, reducing redundancies, enabling real-time monitoring, and improving patient stratification. Integrated systems can cut execution timelines remarkably, boosting cost-efficiency and trial success rates.
- Reduced regulatory risks: Strong data governance and transparent reporting reduce review delays. Unified datasets with automated validation, privacy safeguards, and compliance frameworks (GxP, GDPR) ensure traceability, speed approvals, and prevent costly rework—turning compliance into a competitive advantage.
- Enhanced collaboration: Breaking down pharma data silos creates a single source of truth for R&D, manufacturing, regulatory, and commercial teams, plus partners. Centralized, role-based access speeds knowledge sharing, aligns decisions, and accelerates drug development and market launch success.
Straive in Action: Unlocking the Power of Clinical Data
A global pharma company’s Regulatory Affairs Labeling team was struggling with slow, manual processes. Each year, nearly 20,000 labeling documents had to be classified by type and country, with more than 20 attributes filled in by hand—often pulling information from different systems, languages, and formats. This meant long hours of repetitive work, frequent delays, and a constant risk of human error.
Straive introduced advanced Named Entity Recognition (NER) models built on the company’s own data. These models delivered higher accuracy than generic cloud solutions and were paired with an easy-to-use interface that fit seamlessly into daily workflows. Metadata was standardized to meet company-wide compliance requirements, ensuring that every document was accurate, audit-ready, and consistent.
The initiative helped the company save $5.2 million annually, while cutting the time spent per document from more than 45 minutes to just 2 minutes. It also improved accuracy levels to over 90%, minimized manual errors, and gave the organization stronger confidence in its compliance processes. This example highlights how breaking down data silos in regulatory workflows can free teams from repetitive manual tasks, improve accuracy, and create a more connected, efficient system for managing critical information.
Toward a Seamless, Data-Driven Pharma Future
Straive helps break down pharma data silos for operational agility and better trial and regulatory outcomes. Straive enables this with advanced AI, governance, and integration solutions that unify data across the pharma value chain. The future lies in seamless data ecosystems where real-time insights, predictive analytics, and collaborative platforms accelerate drug development, reduce costs, and bring life-changing therapies to patients faster.
About the Author

Santosh Shevade is a Principal Data Consultant at Gramener – A Straive Company. With deep expertise in healthcare strategy, digital health, and clinical development and operations, he has supported nearly 50 clinical development programs across all clinical phases. His experience spans advanced analytics solution design for pharmaceutical companies, mHealth implementation, and AI applications in healthcare. Previously at Novartis and Johnson & Johnson, Santosh led global clinical development teams and streamlined data review processes for major regulatory submissions. A certified MBTI trainer and leadership coach, he serves as visiting faculty at ISB Hyderabad and Welingkar Institute, focusing on healthcare technology innovation and biopharma strategy.
Share with Friends:
We want to hear from you
Leave a Message
Our solutioning team is eager to know about your
challenge and how we can help.