Pharma Labeling Transformation: SCMS and the Road to Digital Compliance
Posted on: February 16th 2026
Introduction: The Cost of Inaction in Global Pharma Labeling
For many pharmaceutical labeling teams, keeping product information accurate across global markets has become increasingly difficult. Regulatory timelines are tightening, submissions are moving faster, and updates to labeling content are happening more often than before. Yet in many organizations, labeling work is still managed through document-heavy workflows, making it harder to track changes, maintain consistency, and respond quickly to regulatory expectations.
Labeling sits at the center of global coordination, patient safety, and regulatory responsibility. Health authorities such as the FDA, EMA, and PMDA each follow different rules, and those requirements continue to evolve. In day-to-day operations, however, multilingual updates, data checks, and change tracking are often spread across disconnected systems and manual handoffs. This makes it harder for teams to keep labeling information aligned across regions.
The impact of these gaps can be serious. Small inconsistencies in safety language, dosage instructions, or translations can quickly raise regulatory questions or delay approvals. In some cases, a single labeling error is enough to stall a product launch, trigger rework across markets, or create legal and reputational risk for the organization.
Solving these challenges requires more than minor process tweaks. Many pharma organizations are now rethinking labeling as a connected, data-driven operation rather than a collection of documents. This shift brings together structured content, automation, and regulatory intelligence across markets. Straive supports this transition by helping teams move away from document-heavy workflows and toward governed, scalable labeling environments, where labeling data can be created, updated, and reused with greater control as part of a practical path to digital compliance.
Foundational Solution: Elevating Structured Content Management (SCMS)
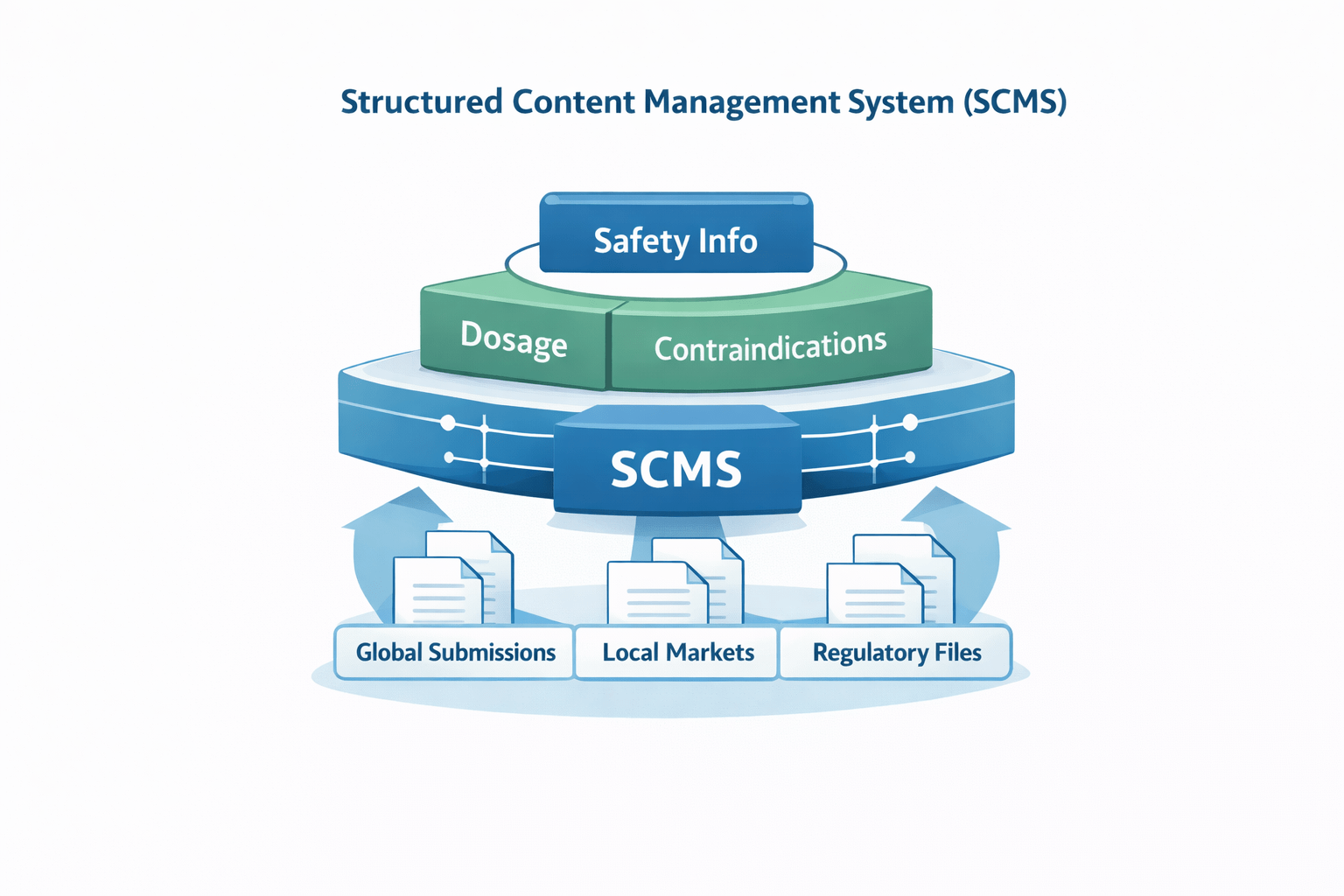
A Structured Content Management System (SCMS) provides the foundation for modern pharmaceutical labeling. Instead of working across disconnected documents, SCMS allows teams to manage labeling information as smaller, reusable content components. Approved content, such as safety statements, dosage instructions, and contraindications, is stored in a single source of truth and reused consistently across global regulatory submissions.
This approach improves both flexibility and compliance in labeling operations. By separating content from format, SCMS allows approved information to be reused across different templates, markets, and languages without losing alignment. When updates are made, changes flow automatically wherever that content is used, reducing the risk of outdated information appearing on labels and helping teams stay aligned with standards such as ISO IDMP (Identification of Medicinal Products).
Straive supports this shift through experience in structured content authoring, data standardization, and modular content management for pharma. With a strong understanding of scientific and regulatory requirements, Straive helps teams move away from static documents and toward data-driven labeling operations. In this setup, SCMS acts as the backbone for automation, localization, and version control, enabling faster updates and stronger audit readiness.
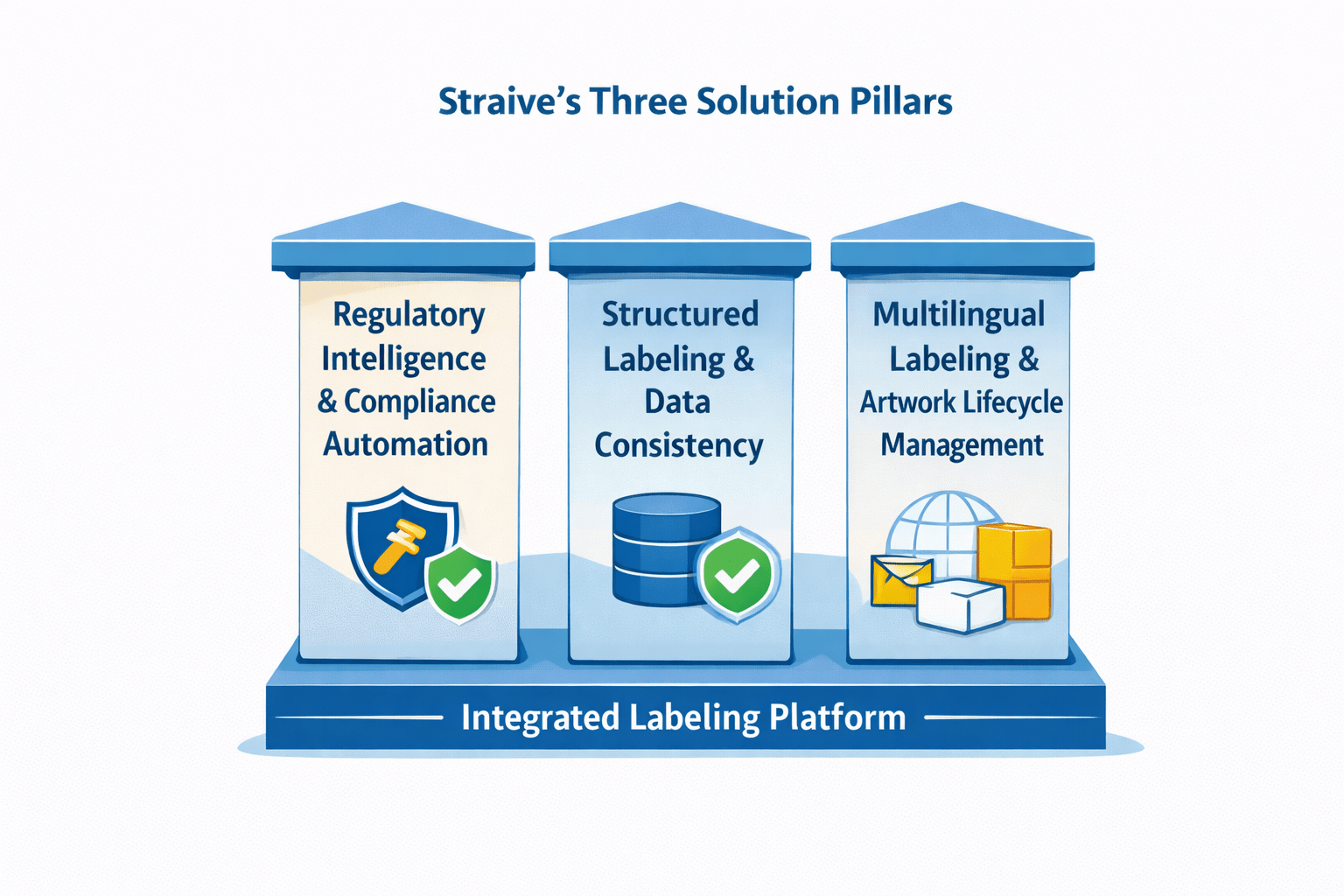
Straive’s Three Solution Pillars for Labeling Transformation
Pillar 1: Regulatory Intelligence and Compliance Automation
Global regulatory expectations for pharmaceutical labeling continue to change, and not always at the same pace. Labeling teams must monitor updates from multiple health authorities, each with different formatting rules, terminology preferences, and timelines for safety revisions. As the volume of changes increases, even small updates can be missed, which often leads to rework, review delays, or slower approvals.
Straive helps teams manage this complexity by bringing regulatory intelligence, structured content, and workflow logic into a single framework. Regulatory updates are monitored and linked to the products they affect, making it easier to see what needs attention early in the process. Automated version tracking shows what changed, when it changed, and why, reducing the effort required to prepare for internal audits or health authority inspections.
Pillar 2: Structured Labeling and Data Consistency
Labeling data rarely comes from a single source. Clinical teams contribute trial information, safety teams monitor signals, and manufacturing teams update batch details. When this information lives in separate systems, inconsistencies start to appear, such as mismatched dosage language or conflicting product information across labels.
To address this, many organizations are moving toward structured labeling models, such as Structured Product Labeling (SPL), where information is stored as smaller, reusable content blocks. Instead of copying and pasting the same text into multiple documents, teams reference a single, approved component that stays consistent wherever it is used.
Straive supports this approach by enabling modular labeling frameworks that track how content is used across products and geographies. When a change is made, the impact is immediately visible wherever that content appears. AI-assisted labeling checks also help flag mismatched or outdated text early in the review process, allowing experts to focus on judgment-based decisions rather than manual document comparison.
Pillar 3: Multilingual Labeling and Artwork Lifecycle Management
Global labeling requires every content change to move across multiple languages and packaging formats at the same time. A single update to approved labeling text can trigger revisions across cartons, leaflets, and blister packs. When these updates are managed manually, translation inconsistencies often appear late in the process, which can delay launches and create additional rework.
Straive supports multilingual labeling workflows that keep translation aligned with regulatory expectations. AI-assisted translation helps maintain consistent terminology, while in-country reviewers remain responsible for linguistic accuracy and local context. Centralized tracking allows teams to see review progress and market readiness in real time, reducing uncertainty across regions.
On the artwork side, Straive connects structured content directly to design files, which reduces manual text re-entry. Digital proofing and automated checks flag issues such as misaligned text or formatting changes before files move to production. This integrated approach helps ensure that every package reflects the most recent approved labeling information.
Direct Call to Partnership: Redefining Global Labeling Excellence with Straive
Pharmaceutical labeling is no longer just a documentation task. It now plays a direct role in how quickly products reach the market and how safely information reaches patients. As labeling requirements become more complex, many organizations look for partners that understand regulatory detail, content automation, and the day-to-day realities of global labeling operations.
Straive supports pharmaceutical teams in moving away from document-heavy processes and toward connected labeling ecosystems. Through its Science and Research Content Solutions, Straive combines three core strengths that help organizations manage labeling with greater confidence and control.
- Pharma domain expertise: Strong understanding of regulatory frameworks, including FDA, EMA, and PMDA requirements, and the documentation needed to support compliance.
- Intelligent automation: Workflow orchestration, content validation, and AI-assisted governance that help reduce review cycles and turnaround time.
- Global multilingual delivery: Proven experience managing localization, translation, and artwork workflows across regions and markets.
Conclusion: A Smarter, Connected Future for Pharma Labeling
The next phase of global pharma labeling will be driven by structured automation and better use of data. Organizations that connect regulatory monitoring, structured content management, and digital artwork workflows will be better prepared to stay compliant and respond quickly to change.
By combining domain expertise with intelligent automation, Straive helps pharmaceutical teams turn labeling into a connected and audit-ready operation. This approach improves accuracy and traceability while supporting continuous compliance, helping ensure that patients across markets receive clear, consistent, and up-to-date information.

Santosh Shevade is a Principal Data Consultant at Gramener – A Straive Company. With deep expertise in healthcare strategy, digital health, and clinical development and operations, he has supported nearly 50 clinical development programs across all clinical phases. His experience spans advanced analytics solution design for pharmaceutical companies, mHealth implementation, and AI applications in healthcare. Previously at Novartis and Johnson & Johnson, Santosh led global clinical development teams and streamlined data review processes for major regulatory submissions. A certified MBTI trainer and leadership coach, he serves as visiting faculty at ISB Hyderabad and Welingkar Institute, focusing on healthcare technology innovation and biopharma strategy.