AI-Powered Translation and Localization in Pharma Content Operations
Posted on: December 22nd 2025
Pharmaceutical organizations have always operated across borders, but today’s environment places unprecedented pressure on how quickly and accurately scientific information must be adapted for global audiences. A single product can generate a cascade of multilingual needs: trial documents for investigators in multiple regions, regulatory materials tailored to local authorities, and patient-facing communication adapted for cultural and linguistic nuance. Errors travel quickly in this ecosystem. A mistranslated contraindication, an ambiguous dose instruction, or an inconsistent adverse-event description does more than disrupt a review cycle, it can reshape clinical outcomes or delay market access.
As multilingual content volumes rise, the weaknesses of traditional translation workflows become increasingly visible. Manual coordination across vendors, reliance on static term bases, and disconnected systems cannot keep pace with the expanding demands of global development programs. Many organizations now recognize that multilingual accuracy is not simply a linguistic requirement but a core operational asset. This shift is why Straive has made AI-enabled pharmaceutical localization a central component of its life sciences solutions, integrating technology, structured content, and regulatory insight to support large-scale multilingual operations with the rigor they require.
The Rising Challenge of Global Translation in Pharma
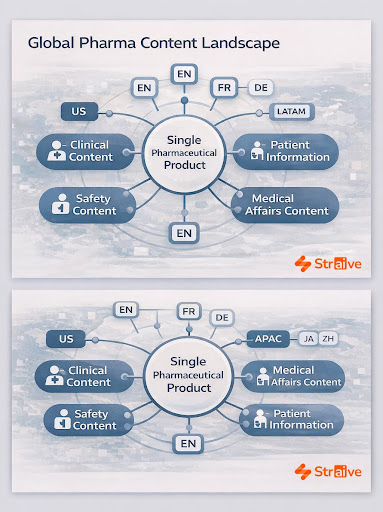
The volume, variety, and regulatory sensitivity of multilingual content in life sciences place unique demands on translation teams. Clinical operations rely on accurate translations for informed consent forms, patient information sheets, investigator brochures, recruitment materials, and safety communications, documents where a single misinterpretation may affect patient understanding or site compliance. Regulatory teams work under similar pressure, adapting SmPCs, product information, labeling components, lifecycle updates, and supporting narratives to the conventions and expectations of individual authorities. Even minor lexical differences can prompt clarification requests from agencies.
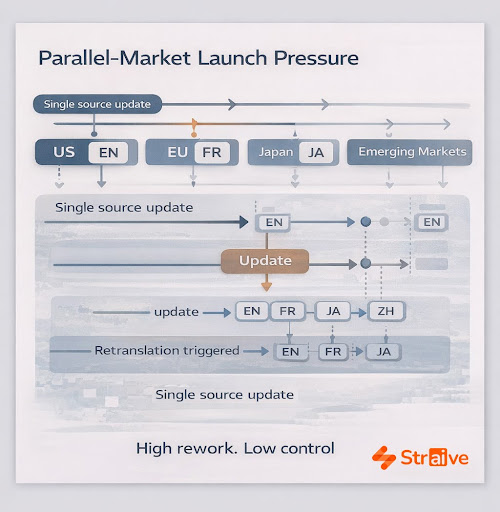
Complicating matters further, global launches increasingly occur in parallel markets, not sequential ones, compressing timelines and expanding linguistic complexity. Teams frequently manage dozens of language versions at once, with every update triggering a new wave of translations. Generic models struggle to maintain metadata alignment, structured labeling conventions, or therapeutic nuance. This is where Straive’s capabilities begin to matter, because multilingual precision in pharma depends on deep familiarity with regulatory structures, therapeutic taxonomies, and scientific meaning, not just linguistic fluency.
Straive’s multilingual frameworks support clinical, regulatory, safety, and medical affairs teams by coupling structured content principles with advanced AI and domain-trained linguists. This integrated approach reduces drift across versions, shortens turnaround times, and ensures that every language reflects the same scientific intent.
Why Traditional Translation Models Fall Short
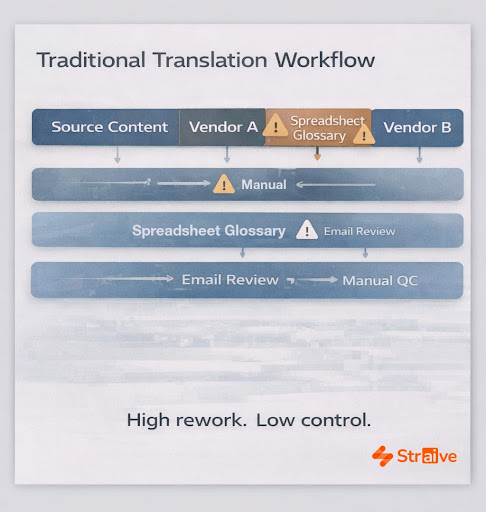
Conventional translation workflows rely on manual processes that are difficult to scale and even harder to control. Static glossaries and spreadsheets cannot accommodate the level of precision required in therapeutic domains where terminology evolves and local labeling expectations vary. Multiple CROs and language vendors may apply different interpretations, creating inconsistencies that must be reconciled through time-consuming reviews. These inefficiencies increase risk, especially during late-stage clinical activity or regulatory submissions where version alignment is non-negotiable.
Generic machine translation tools introduce their own limitations. While acceptable for routine content, they are not designed to interpret complex scientific language, structured sections of labeling, adverse-event terminology, or protocol-specific nuances. They also lack the ability to preserve metadata or manage structured fields that are essential in pharma workflows.
Straive addresses these gaps with a domain-trained approach to AI. Its models are built on biomedical, clinical, and regulatory data, enabling contextual comprehension that generic systems cannot achieve. This scientific grounding ensures that translation output aligns not only with linguistic standards but with the expectations of regulatory authorities and therapeutic conventions. More importantly, Straive’s frameworks integrate translation with upstream and downstream workflows, an essential difference when managing content across global markets and multiple functions.
Inside AI-Powered Translation and Pharmaceutical Localization
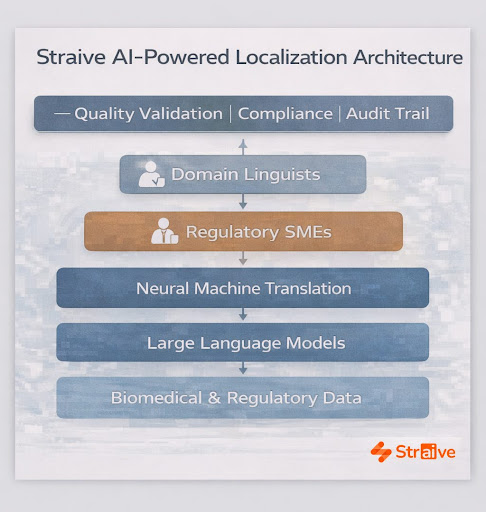
Modern AI-enabled translation ecosystems bring together neural machine translation, large language models, terminology governance, and structured content management. The value lies not in automation for its own sake, but in the ability to interpret meaning through regulatory logic, scientific terminology, and therapeutic context.
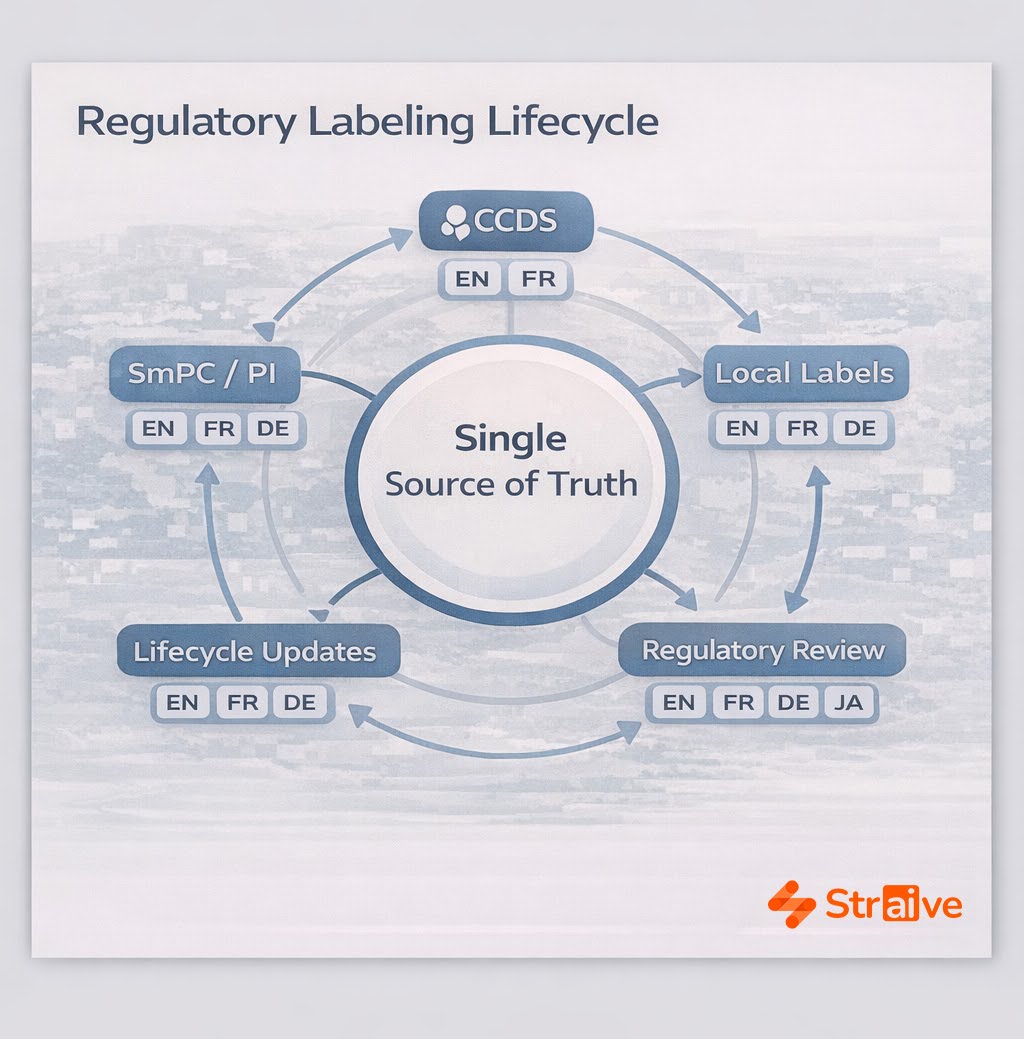
Straive’s translation architecture uses pharma-specific glossaries, ontologies, and labeling taxonomies to anchor consistency across all language versions. These resources are continuously refined by domain experts, ensuring that AI output aligns with CCDS terminology, SmPC conventions, risk communication standards, and market-specific regulatory structures. Automated quality controls validate terminology usage, metadata tagging, bilingual equivalence, and structural alignment.
Yet AI alone is not enough. High-stakes content, especially regulatory, clinical, and safety documentation, requires human expertise. Straive’s linguists and regulatory SMEs perform scientific NMT post-editing, ensuring that translations preserve meaning at the level regulators expect. Their involvement also strengthens the AI models themselves, creating a cycle where machine learning benefits from human insight, and human accuracy benefits from machine-level scale.
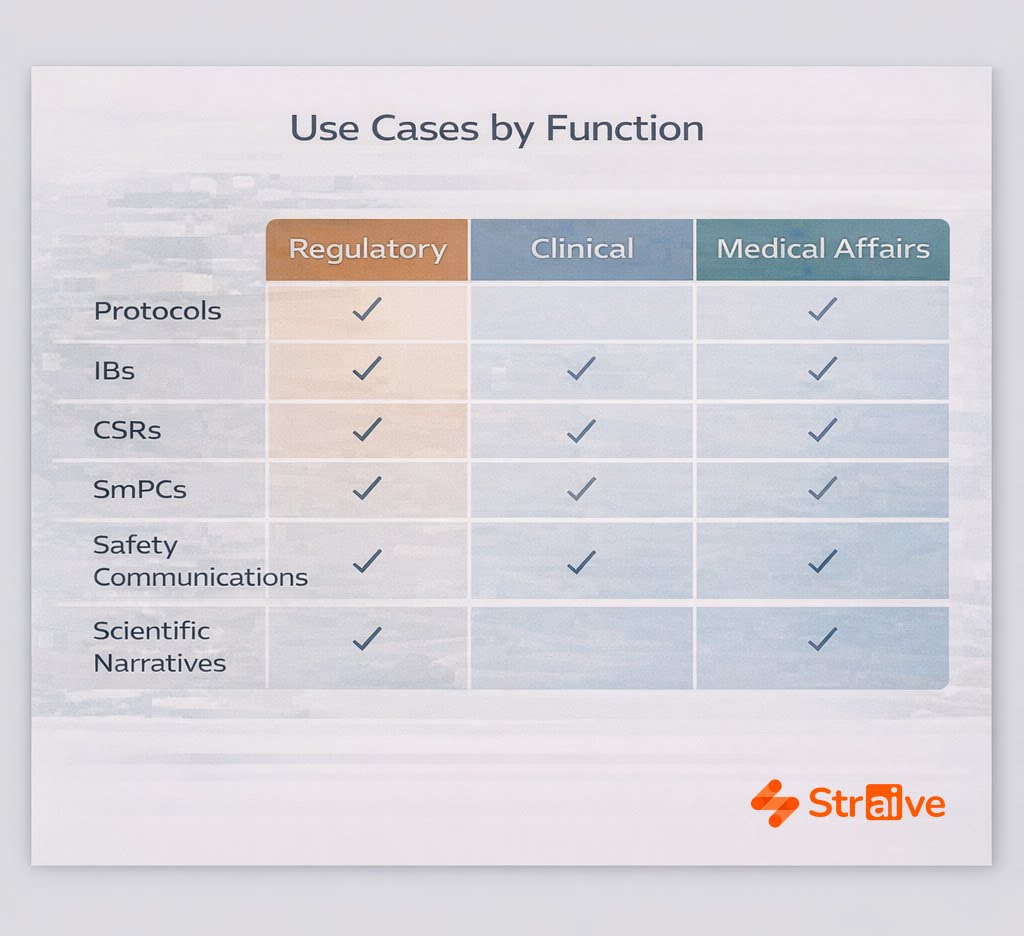
Use Cases Across Pharma Content Workflows
Regulatory Submission Translation
Regulatory submissions demand precise, synchronized translations of SmPCs, product information, CCDS updates, and related components. Straive’s models accelerate this work by ensuring consistent terminology across markets and by integrating translation workflows with labeling lifecycle processes. This reduces deviations and keeps multilingual documents aligned throughout submission cycles.
Clinical Documentation
Protocols, IBs, CSRs, safety communications, and eConsent materials must be translated quickly and accurately to support trial startup and ongoing operations. Straive’s AI-guided workflows maintain metadata consistency and therapeutic terminology across languages, enabling faster site activation and reducing rework.
Medical Affairs Localization
Scientific narratives for HCPs, MSLs, and field medical teams require adaptation that preserves both scientific accuracy and local relevance. Straive’s linguists and SMEs ensure that content reflects regional expectations while maintaining the integrity of the scientific message.
Across all use cases, organizations see measurable impact: faster turnaround, fewer discrepancies, reduced retranslation cycles, and improved global consistency, outcomes enabled by Straive’s integrated, domain-aware translation ecosystem.
Ensuring Compliance, Traceability, and Data Security
Compliance cannot be an afterthought in multilingual pharma operations. Every translated document must withstand scrutiny from regulators who expect traceability across versions and transparent rationale for linguistic decisions. Straive’s AI-enabled workflows are built with GxP and ISO expectations in mind, ensuring that each translation action, whether machine-generated, post-edited, or approved, is captured in a complete audit trail.
Data security is equally essential. With sensitive clinical and regulatory content moving through multilingual pipelines, Straive enforces strict encryption, secure access controls, and protected translation environments. Its explainable AI approach enables teams to understand why a translation was produced and how terminology or metadata rules were applied, reinforcing trust during internal audits or regulatory inspections.
Human + AI Collaboration for High-Stakes Localization
High-quality translation depends on the synergy between technology and human expertise. AI handles scale, enforces terminology, and maintains metadata alignment. Human experts interpret therapeutic nuance, validate scientific accuracy, and ensure contextual relevance. Straive’s model brings together regulatory SMEs, clinical linguists, AI engineers, and workflow specialists, creating a feedback loop where each component strengthens the others. This fusion is what enables reliability across therapeutic areas, document types, and global markets.
How Straive Enables Intelligent Multilingual Pharma Operations
Straive supports global life sciences organizations with an end-to-end multilingual ecosystem that integrates AI, structured content management, terminology governance, and scientifically validated post-editing. Its translation workflows align with labeling lifecycle management, clinical operations, regulatory submissions, and safety communication, ensuring consistency across the entire value chain.
By combining domain-trained AI with SME oversight, Straive delivers accuracy that meets regulatory expectations while accelerating cycle times and reducing localization costs. Organizations gain a unified, scalable multilingual platform that supports clinical, regulatory, safety, and medical affairs content within a single, intelligence-driven model.
Conclusion — The Future of Global Pharma Communication
The industry is moving toward multilingual operations that are intelligent, connected, and built for regulatory precision. Manual translation pipelines can no longer support the scale and complexity of global pharmaceutical development. AI-powered translation—guided by structured content and domain expertise, offers a practical and sustainable path forward.
Straive enables this transition by integrating linguistic intelligence, regulatory knowledge, and scalable technology frameworks. The future of pharma content will be multilingual by design, and Straive is helping organizations build the ecosystems required to achieve global consistency, scientific accuracy, and operational excellence.

Santosh Shevade is a Principal Data Consultant at Gramener – A Straive Company. With deep expertise in healthcare strategy, digital health, and clinical development and operations, he has supported nearly 50 clinical development programs across all clinical phases. His experience spans advanced analytics solution design for pharmaceutical companies, mHealth implementation, and AI applications in healthcare. Previously at Novartis and Johnson & Johnson, Santosh led global clinical development teams and streamlined data review processes for major regulatory submissions. A certified MBTI trainer and leadership coach, he serves as visiting faculty at ISB Hyderabad and Welingkar Institute, focusing on healthcare technology innovation and biopharma strategy.