AI Meets Domain Expertise: How Straive Builds Pharma Publishing Pipelines That Scale
Posted on: August 13th 2025
In pharmaceutical and life sciences publishing, speed and accuracy are everything. From clinical trials to regulatory approval, every piece of content plays a critical role in shaping medical narratives, driving drug adoption, and ensuring compliance. But today’s pharma publishing ecosystem is fragmented—marked by time-intensive processes, siloed systems, and growing regulatory demands.
At the intersection of these challenges lies an opportunity: to reimagine pharma content pipelines by integrating AI with deep domain expertise. This is precisely where Straive delivers transformational value.
The Pressure to Publish Smarter—and Sooner
The demand for faster, high-quality publications in pharma is only increasing. Clinical trial sponsors, medical affairs teams, and regulatory writers all face tight timelines—from first patient in (FPI) to marketing authorization. But delays are common. Manuscripts get bounced between internal reviewers, external authors, medcom agencies, and journal publishers—each with their own systems and standards.
Meanwhile, regulators and journals now expect full transparency, rigorous data disclosure, and adherence to evolving guidelines such as GPP 2022, ICMJE, and CONSORT. This puts publishing teams under enormous pressure to ensure accuracy, compliance, and speed—simultaneously.
Why Life Sciences Publishing Needs a Specialized Approach
Publishing in life sciences is markedly different from STM or academic publishing. A single article may need to align with brand strategy, clinical findings, regulatory positions, and ethical transparency. Minor errors in citations, disclosures, or author contributions can raise red flags with journals or health authorities.
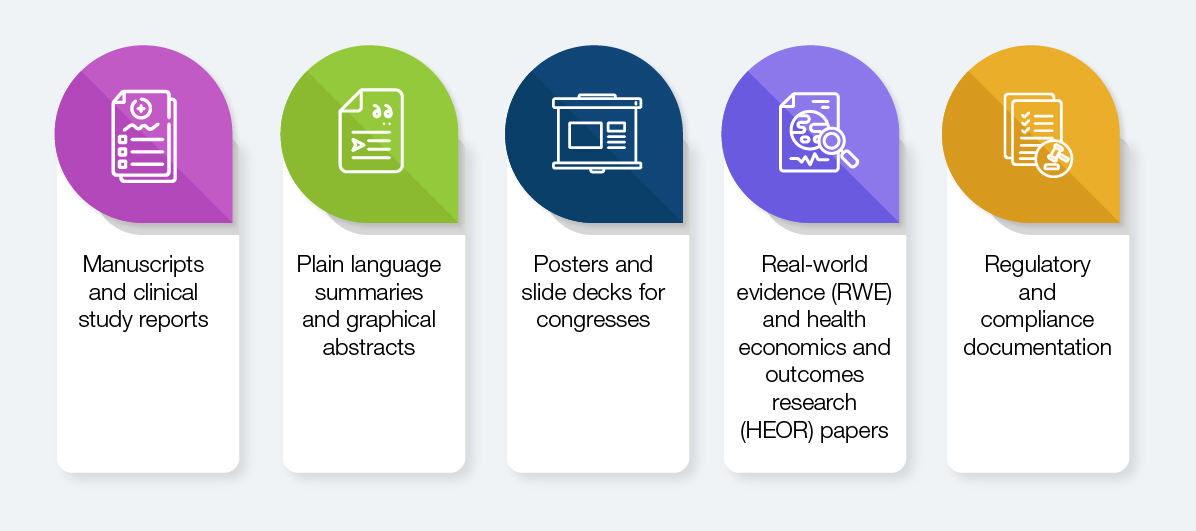
Pharma Pipelines Produce Diverse Content Across Scientific and Compliance Domains
Each of these has distinct formatting, referencing, disclosure, and data presentation needs. A one-size-fits-all model doesn’t work. What’s needed is a structured, intelligent, and deeply contextual publishing solution—precisely what Straive offers.
Where AI Delivers—and Where It Needs Domain Expertise
AI is already making publishing faster. Natural Language Processing (NLP), content enrichment tools, and machine learning models are used to:
- Extract trial and author metadata.
- Standardize references and citations.
- Enhance grammar and language.
- Tag content elements for submission.
- Auto-format based on journal guidelines.
However, without domain expertise, AI can fall short—misclassifying trial types, misunderstanding terminology, or missing subtle but critical context (e.g., differentiating between a treatment arm and a control group in trial data).
At Straive, we believe AI should augment, not replace, human expertise. Our approach integrates AI into human-in-the-loop frameworks that enable speed and precision.
End-to-End Acceleration: From Authoring to Omnichannel Delivery
Straive has redefined what pharma publishing workflows can look like. Through structured frameworks and domain-aware AI engines, we help pharma clients reduce cycle times, increase throughput, and maintain high levels of compliance.
Key solutions include:
- Structured Content Authoring (SCA) Straive enables modular, template-driven content creation where sections of a manuscript—such as methods, disclosures, or data visualizations—can be generated, validated, and reused across documents. This modularity reduces rework and maintains consistency across teams, geographies, and brands.
- Content Transformation and Multi-Format Publishing We support clients in transforming content into multiple formats: journal submissions, XML outputs, patient-centric formats like plain language summaries, and repurposed digital assets for congresses and field use. This includes compatibility with platforms like PubMed Central, CrossRef, and regulatory portals.
- AI-Powered Content Enrichment Using proprietary tools like aiKira, Straive auto-tags biomedical entities, extracts relationships, enhances metadata, and ensures that content is submission-ready. Our AI is fine-tuned on domain-specific corpora to understand therapeutic nuances and medical language.
- Review and Quality Automation Automated quality checks ensure that manuscripts conform to journal-specific formats, detect missing disclosures, flag inconsistencies, and support anti-plagiarism and de-duplication workflows. Experienced medical editors oversee all of this, ensuring both automation and accountability.
Straive’s Domain-Aligned Model for Scalable Pharma Publishing
Straive brings decades of experience supporting science and research publishing, and we’ve extended that domain excellence to the life sciences sector with tailored services for pharma and biotech clients.
Here’s what sets us apart:
- Therapeutic Expertise Across Functions Our publishing teams include MDs, PhDs, and therapeutic-area specialists across oncology, neurology, cardiology, immunology, and rare diseases. We pair this medical depth with operational scalability, enabling clients to scale content production across brands and indications.
- Publishing Technology That Integrates with You Straive’s platforms and automation tools are designed to plug into your ecosystem—whether you’re using Veeva Vault, Datavision, or other proprietary medical writing or content management platforms. We ensure minimal disruption and maximum synergy.
- Compliance-First Mindset We follow GPP, ICMJE, and ICH guidelines rigorously and apply quality standards certified by ISO 9001 and ISO 27001. Our workflows are designed with auditability and traceability in mind, giving your regulatory and compliance teams confidence.
- Global Operations, Local Sensitivity With teams across North America, Europe, and Asia-Pacific, Straive supports global publications strategies with local nuance—whether that means region-specific formatting, multilingual content creation, or in-market regulatory alignment.
The Road Ahead: Pharma Publishing as a Strategic Enabler
Publishing is no longer just an endpoint in the pharma communication lifecycle—it’s a strategic enabler that influences everything from market readiness to scientific reputation.
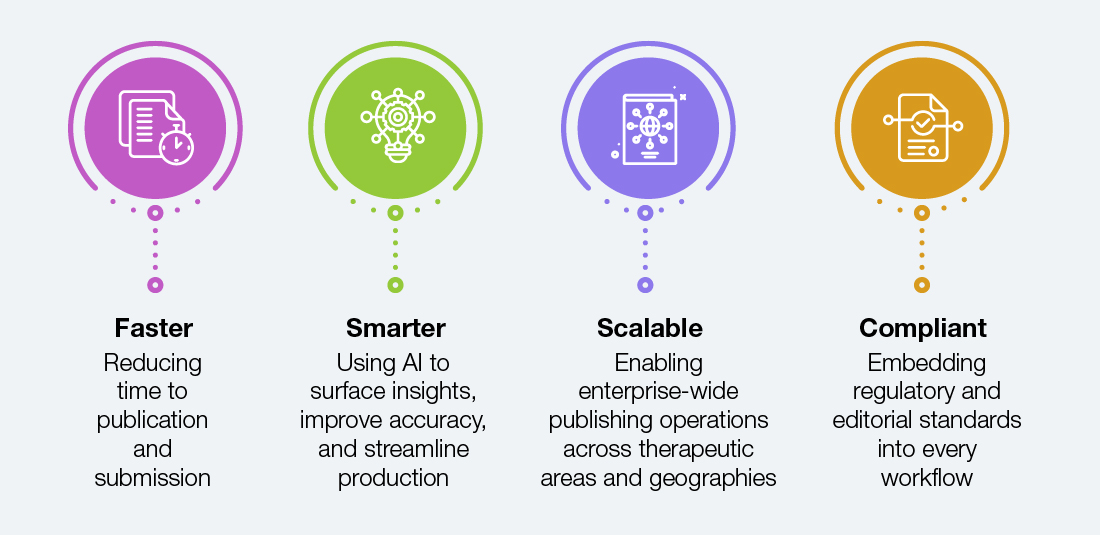
Straive Envisions Publishing Pipelines That Are Faster, Smarter, Scalable, and Compliant
As pharma companies prepare for more decentralized trials, diverse evidence formats, and multichannel publishing demands, Straive stands ready to lead the transformation—with a unique blend of automation, domain expertise, and editorial excellence.
The future of pharma publishing isn’t just digital—it’s intelligent, agile, and domain-first. And Straive is building that future, one publication at a time.
Looking to transform your publication pipeline? Schedule a tailored demo with our experts to see how Straive’s AI-powered workflows can accelerate your medical content delivery—from authoring to submission.
About the Author

Santosh Shevade is a Principal Data Consultant at Gramener – A Straive Company. With deep expertise in healthcare strategy, digital health, and clinical development and operations, he has supported nearly 50 clinical development programs across all clinical phases. His experience spans advanced analytics solution design for pharmaceutical companies, mHealth implementation, and AI applications in healthcare. Previously at Novartis and Johnson & Johnson, Santosh led global clinical development teams and streamlined data review processes for major regulatory submissions. A certified MBTI trainer and leadership coach, he serves as visiting faculty at ISB Hyderabad and Welingkar Institute, focusing on healthcare technology innovation and biopharma strategy.
Share with Friends:
We want to hear from you
Leave a Message
Our solutioning team is eager to know about your
challenge and how we can help.